Abstract
Background: High grade B-cell Lymphoma with rearrangements of MYC and BCL2 and/or BCL6, also known as double hit lymphoma (DHL), and double expressing lymphomas (DEL; DLBCL with IHC expression of MYC and BCL2, but without double hit cytogenetics) are highly aggressive B cell non-Hodgkin lymphomas and patients (pts) often develop relapsed/refractory disease following standard first-line chemoimmunotherapy. DA-EPOCH-R is widely used in patients with DHL, and a phase 1 trial of venetoclax + DA-EPOCH-R established the dose of 600 mg for 5 days with each cycle as the recommended phase 2 dose (Rutherford, et al. Proc ASCO 2020). A051701 is a phase II/III randomized trial evaluating chemoimmunotherapy +/- the BCL2 inhibitor venetoclax in a DHL cohort (pts with DHL with BCL2 rearrangement and/or expression), and a DEL cohort. Here we report the initial results from the DHL cohort of A051701.
Methods: Pts age ≥ 18 years with newly diagnosed DHL were stratified on International Prognostic Index (IPI) score and receipt of a single cycle of chemotherapy prior to registration, which was permitted, and randomized 1:1 to receive DA-EPOCH-R (Arm 1) or DA-EPOCH-R with venetoclax (Arm 2). Enrollment was based on local pathology results which were centrally confirmed. DA-EPOCH-R was administered as previously published. Venetoclax was dosed at 600 mg po daily on days 4-8 of cycle 1 and on days 1-5 of subsequent cycles for up to 6 total cycles. All cycles were supported by GCSF or peg-GCSF. For the interim analysis at the end of phase II, 53 events ensured 90% power to detect an improvement in 24-month progression-free survival (PFS) from 40% in Arm 1 to 60% in Arm 2 (HR=0.557) using a one-sided stratified log-rank test with type I error rate of 20%. The Alliance Data and Safety Monitoring Board approved the data release after accrual was stopped early due to excess toxicity in Arm 2. These data were frozen July 8, 2021.
Results: 73 pts were registered (36 in Arm 1 and 37 in Arm 2) between 8/7/19 and 9/18/20. Analyses were performed for the modified intent-to-treat (mITT) population, defined as eligible pts with DHL histology confirmed (n=66; 30 Arm 1, 36 Arm 2). Median age was 65 years in both arms (range 37-80) and baseline demographic factors were well balanced. An allowed pre-protocol chemoimmunotherapy cycle was administered in 60% and 56% of Arm 1 and Arm 2 pts, respectively. The majority of pts had advanced stage disease (Arm 1 87%, Arm 2 86%), GCB immunophenotype (Arm 1 100%, Arm 2 88%), ECOG PS 0-1 (Arm 1 90%, Arm 2 86%), and high-intermediate/high risk IPI score (Arm 1 63%, Arm 2 64%). Therapy was completed per protocol in 70% and 47% of pts, respectively, for Arm 1 and 2, and disease progression on therapy occurred in 10% and 3%. Treatment was discontinued due to adverse events in 7% of pts in Arm 1 and 11% of pts in Arm 2. Consent was withdrawn after beginning treatment in 3% and 11% in Arm 1 and 2, respectively. Death on treatment occurred in 1 patient (3%; PJP pneumonia) in Arm 1 and 6 pts (17%; sepsis (n=4), cardiac arrest (n=2)) in Arm 2. The observation of excess deaths on Arm 2 prompted suspension of accrual to the DHL cohort. Treatment was discontinued for other reasons in 3 (10%) and 4 (11%) pts in Arms 1 and 2, respectively. Venetoclax was dose-reduced and/or omitted in 15 (42%) pts. Grade 4 neutropenia was not significantly different between arms (Arm 1 67%, Arm 2 71%), nor was grade ≥3 neutropenic fever (Arm 1 36%, Arm 2 40%). Sepsis occurred more frequently in Arm 2 (14% vs. 23%).
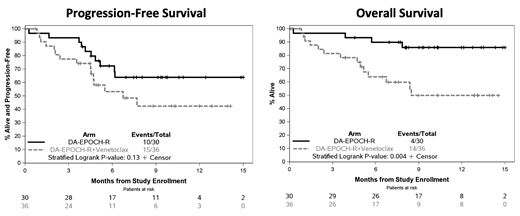
The end of treatment overall and complete response rates in the mITT population were 73% and 67% in Arm 1, and 58% and 50% in Arm 2. The end of treatment overall and complete response rates among only pts evaluated for response (n=52) were 79% and 71% in Arm 1, and 88% and 75% in Arm 2. With a median follow-up of 7.4 months, the median PFS was not reached (NR) in Arm 1 (95% CI 6.1-NR) and was 6.7 months in Arm 2 (95% CI 4.5-NR) (two-sided p=0.13). The median OS was NR in Arm 1 and was 8.5 months in Arm 2 (95% CI 5.2-NR) (two-sided p=0.004).
Conclusions: DA-EPOCH-R plus venetoclax in DHL pts resulted in excess treatment-related mortality compared to DA-EPOCH-R alone, prompting early discontinuation of accrual and venetoclax treatment for the study cohort. Based on these data, we recommend against combining venetoclax with DA-EPOCH-R in DHL. Robust accrual to this study shows that prospective trials are feasible in DHL, and the favorable outcome thus far in the DA-EPOCH-R arm may serve as a benchmark for future trials
Abramson: Astra-Zeneca: Consultancy; Allogene Therapeutics: Consultancy; Incyte Corporation: Consultancy; BeiGene: Consultancy; Kymera: Consultancy; Bluebird Bio: Consultancy; Genmab: Consultancy; EMD Serono: Consultancy; Bristol-Myers Squibb Company: Consultancy, Research Funding; C4 Therapeutics: Consultancy; Morphosys: Consultancy; Kite Pharma: Consultancy; Novartis: Consultancy; Seagen Inc.: Research Funding; AbbVie: Consultancy; Karyopharm: Consultancy; Genentech: Consultancy. Ruppert: Telios Pharma: Consultancy. Hsi: Seattle Genetics: Honoraria; Cytomx: Honoraria; Eli Lilly: Research Funding; AbbVie: Research Funding. Landsburg: Triphase: Research Funding; Takeda: Research Funding; Curis: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Other: DSMB member; ADCT: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees. Kahl: AbbVie, Adaptive, ADCT, AstraZeneca, Bayer, BeiGene, Bristol-Myers Squibb, Celgene, Genentech, Incyte, Janssen, Karyopharm, Kite, MEI, Pharmacyclics, Roche, TG Therapeutics, and Teva: Consultancy; AbbVie, Acerta, ADCT, AstraZeneca, BeiGene, Genentech: Research Funding. Friedberg: Acerta: Other: DSMC ; Novartis: Other: DSMC ; Bayer: Other: DSMC . Bartlett: Millennium: Research Funding; Janssen: Research Funding; Kite, a Gilead Company: Research Funding; Merck: Research Funding; Genentech: Research Funding; Celgene: Research Funding; Forty Seven: Research Funding; Bristol Myers Squibb: Research Funding; Roche/Genentech: Consultancy; Autolus: Research Funding; Seagen: Consultancy, Research Funding; Pharmacyclics: Research Funding; ADC Therapeutics: Consultancy, Research Funding. Leonard: ADC Therapeutics, AstraZeneca, Bayer, BMS/Celgene, Epizyme, Inc., Genmab, Gilead/Kite, Karyopharm, BMS/Celgene, Regeneron, MEI Pharma, Miltenyi, Roche/Genentech, Sutro: Consultancy; Roche/Genentech: Consultancy.
venetoclax in double hit lymphoma
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal